Frequently Asked Questions
1. Why is SUDC Research Important?
At this time, SUDC is neither predictable nor preventable. Research improves our understanding of sudden deaths in children and impacts our ability to prevent them. At the SUDCRRC, we study each child in detail and utilize the expertise of our many team members to learn more as a group than we can as individual researchers. Each multidisciplinary case review of a child teaches us and fosters new research questions and subsequent experiments. We are grateful to the families who allow us to learn from their life’s biggest tragedy. Our common goal is to understand and prevent SUDC.
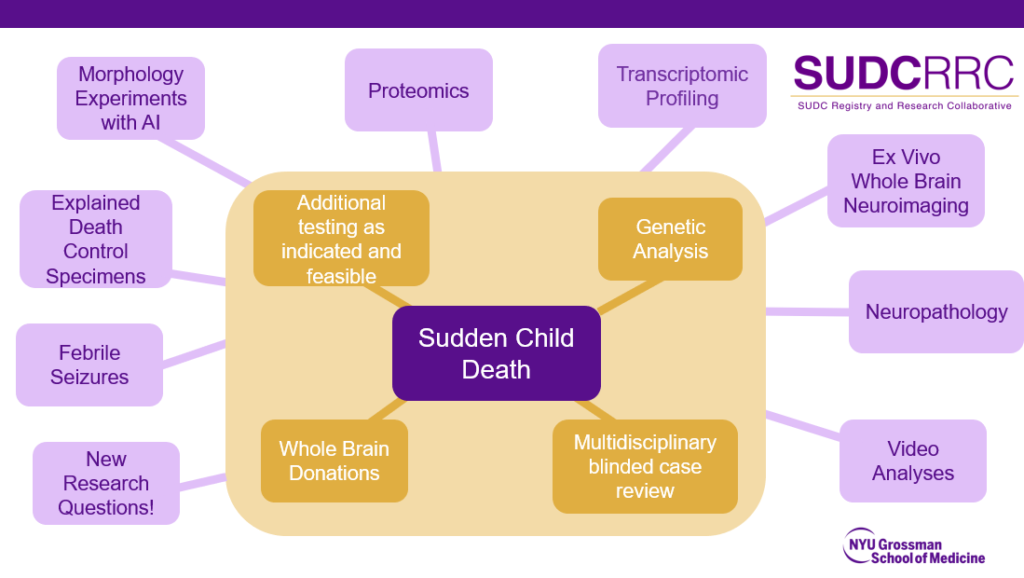
2. How does a family enroll their child? How does a professional refer a family for enrollment?
A family, or professional who is referring a family, can reach us by phone, email, or our contact us form. A study team member will then make contact. If the child satisfies all enrollment criteria and the parent(s) agrees to enrollment, we will mail the parents all study enrollment forms, schedule an enrollment call, and answer any questions they may have.
3. Will families or professionals incur any costs with study enrollment?
No, families and professionals will not incur any costs for study participation. Scientific grants and private donations support the SUDCRRC. We are grateful to the ongoing support of the SUDC Foundation, SUDC-UK and FACES.
4. What does study participation for a family look like?
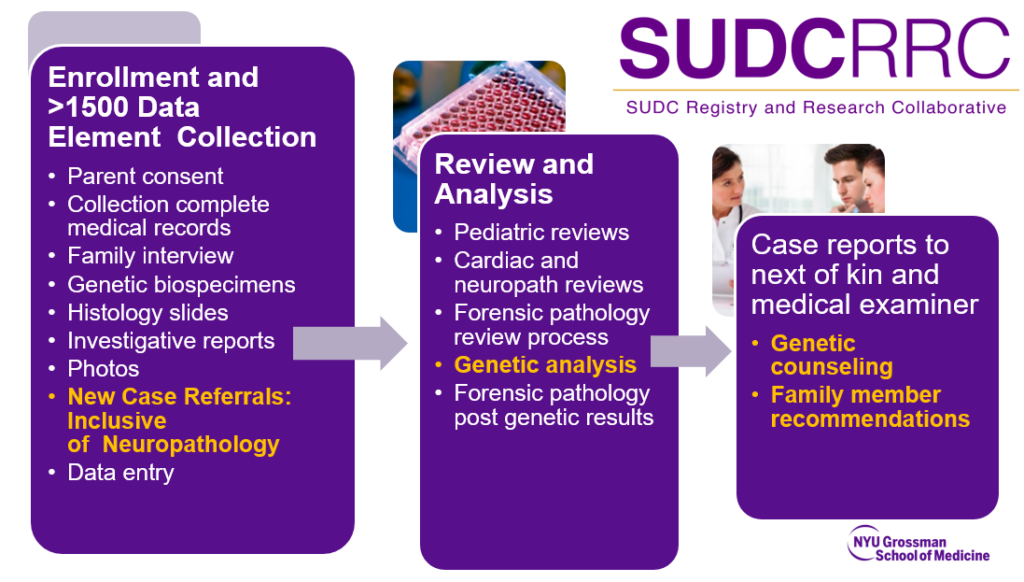
- Participation in the study first involves completing the informed consent process. This includes completing consent forms and authorizations for release of medical information. This is completed by phone or video call with Laura Gould (Coinvestigator and Research Scientist) to ensure that all questions are addressed and the family understands their decision.
- Once enrolled, the parent(s) will complete a family interview and biological parents are asked to provide a blood sample for genetic analysis. In parallel, study team members collect the child’s medical records, and work with the medical examiner/coroner for information and specimens needed to start our research and multidisciplinary review process.
- If parents consent to receive results back from the study, study team members will contact parents as soon as they are available. Time for results vary for each investigation. Families are usually in active participation with us for a few years.
- The case review process is summarized below:
5. Why have I never heard of SUDC before?
SUDC was first defined in 2005. Prior to 1989, most SUDC toddler-aged deaths would likely have been considered Sudden Infant Death Syndrome (SIDS). Annually, more than 400 U.S. children, aged 1-18 years, are affected by unexplained deaths. Similar rates of SUDC occur in the United Kingdom, and likely elsewhere. Awareness and research has been limited, but is growing. Participation in SUDCRRC helps us to learn more and help the medical community to prevent such deaths in the future.
6. Is SUDC the same as SIDS?
By definition, Sudden Unexplained Death in Childhood (SUDC) is different from SIDS (Sudden Infant Death Syndrome) by age. SIDS is characterized by the unexplained death of infants under the age of 12 months, while SUDC is characterized as those deaths occurring between 12 months and 18 years of age that are unexplained after thorough investigation.
SUDC is distinct from Sudden Infant Death Syndrome (SIDS) by a comparative paucity of research and funding. In recent decades, SIDS research has been allocated ~500 million dollars in public funding while no targeting funding for SUDC has occurred in same time period (NIH Estimates of Funding for Various Research, Condition, and Disease Categories, 2023). SIDS cases have declined significantly since 1990 from 130.3 death per 100,000 births to 38.4 deaths per 100,000 births in 2020 (CDC/NCHS, National Vital Statistics, 2020).
7. Are there support systems for grieving families?
Yes, the SUDC Foundation and the SUDC-UK are available to families affected. We encourage families to consider connecting with these resources as well as additional local resources that may be available to them.
8. How is the SUDCRRC funded?
The SUDC Registry and Research Collaborative is supported by NYU F.A.C.E.S., scientific grants from the SUDC Foundation, SUDC-UK, and private donations. Additional research funds are crucial to advance SUDC research and stop these tragedies. If you would like to support our efforts, please see our Support page.

NYU Langone Health
Comprehensive Epilepsy Center
223 E 34th St, Ground Floor
New York, NY 10016

The SUDCRRC is approved by the NYU Langone Health’s Institutional Review Board (i14-01061).